A proprietary formulation was designed that consist of minerals
(zinc, magnesium, iron, calcium, selenium, and copper), vitamins
(pyridoxine HCl, cyanocobalamin, ascorbic acid, alpha tocopherol, and
cholecalciferol), Panax ginsengextract, β-carotene, and
cannabidiol isolate. The present study was aimed to evaluate the impact
of Consciousness Energy Healing Treatment (the Trivedi Effect®) on a novel test formulation in male Sprague Dawley (SD) rats, fed with vitamin D3
deficiency diet (VDD) for antiaging/cognitive and anti-inflammatory
activities. The test formulation was divided into two parts. One part
was denoted as the untreated test formulation without any Biofield
Energy Treatment, while the other part was defined as the Biofield
Energy Treated sample, which received the Biofield Energy Healing
Treatment by renowned Biofield Energy Healer, Mr. Mahendra Kumar
Trivedi. The level of Klotho protein (anti-aging biomarker) in
cerebro-spinal fluids (CSF) was significantly increased by 44.2%, 92.0%,
44.2%, and 43.1% in the Biofield Energy Treatment per se to animals from day -15 (G6), Biofield Energy Treated test formulation from day -15 (G7), Biofield Energy Treatment per seplus Biofield Energy Treated test formulation from day -15 (G8), and Biofield Energy Treatment per seanimals
plus untreated test formulation (G9) groups, respectively as compared
to the disease control group (G2). The level of β-endorphin in CSF
(cognition, pain and inflammation biomarker) was significantly increased
by 418.4%, 1155.7% (p≤0.01), 890.4% (p≤0.01), 351%, and 566.7% in the
G5, G6, G7, G8, and G9 groups, respectively as compared to the G4.
Moreover, serotonin level in CSF was increased by 94.8%, and 63.4% in
the G6 and G9 groups, respectively as compared to the G4. The level of
1, 25 (OH)2D3 in CSF was significantly increased
by 61.8%, 33.4%, 61.5%, 64.5%, and 30.6% in the G5, G6, G7, G8, and G9
groups, respectively as compared to the VDD induced group (G2). Further,
the level of c-reactive protein (CRP, inflammation biomarker) in serum
was reduced by 21.2%, 23.1%, 19.8%, 22.4%, and 23.1% in the G5, G6, G7,
G8, and G9 groups, respectively as compared to the G2 group. Altogether,
results suggested that the Biofield Treated test formulation and
Biofield Energy Treatment per se significantly increased antiaging,
cognitive, and anti-inflammatory biomarkers that could be helpful in
various aging/psychiatric or inflammatory disorders. Thus, the results
showed a significant slowdown of disease progression and all other
disease-related complications/symptoms in the preventive Biofield Energy
Treatment group per seand the Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) as compared to the disease control group.
Keywords: Biofield Treatment;antiaging; The Trivedi Effect®;klotho;β-endorphin;serotonin; Vitamin D3 deficiency diet;calcitriol
Introduction
Deficiency of vitamin D3 is directly linked to various health
problems like osteoporosis, cognitive decline, cardiovascular disease,
depression, diabetes, hypertension, and cancer [1,2]. Vitamin D is very
essential for bone health in adults and children. Its sufficient
concentration prevents osteomalacia, muscle weakness, and protect
fractures. The processes by which intake of vitamin D3 like
synthesis through skin via UV-rays and absorption from foods become less
efficient with age [3]. Hence, hypovitaminosis of vitamin D3
is more prevalence worldwide [4]. Based on this situation authors
constructed the current research work to evaluate the impact of
Consciousness Energy Healing Treatment on aging after induction of
Vitamin D3Deficiency Diet (VDD) in Sprague Dawley rats. The
newly formulated test formulation, which is a combination of multiple
minerals (iron, copper, zinc, magnesium, calcium, and selenium),
vitamins (ascorbic acid, cholecalciferol, pyridoxine HCl, alpha
tocopherol, and cyanocobalamin), panax ginseng extract, and cannabidiol
isolate. Each component of this test formulation commonly used as
nutraceutical supplement [5-8]. Biofield Therapy (or Healing Modalities)
is one of the approach of Complementary and Alternative Medicine (CAM)
therapies now considering as the first-line model of treatment against
several disorders. Based on the obtained data from National Health
Interview Survey (NHIS) 2012, reported that most of the Americans used
the dietary supplement as complementary health approaches than
conventional medicine therapy. Besides, The National Center of
Complementary and Integrative Health (NCCIH) has recognized and accepted
Biofield Energy Healing as a CAM health care approach in addition to
other therapies, medicines and practices such as Tai Chi, Qi Gong,
Ayurvedic medicine, Rolfing structural integration, deep breathing,
yoga, natural products, chiropractic/osteopathic manipulation, massage,
meditation, relaxation techniques, aromatherapy, acupuncture,
progressive relaxation, hypnotherapy, healing touch, mindfulness,
special diets, naturopathy, homeopathy, guided imagery, acupressure,
traditional Chinese herbs and medicines, pilates, movement therapy,
Reiki, essential oils, cranial sacral therapy and applied prayer.
Human Biofield Energy has subtle energy that can work
effectively [9]. CAM therapies have been practiced worldwide
with reported clinical benefits in different health disease profiles
[10]. This energy can be harnessed and transmitted by individuals
into living and non-living things via the process of Biofield Energy
Healing. Biofield Energy Treatment (the Trivedi Effect®) has been
published in numerous peer-reviewed science journals with
significant outcomes in many scientific fields such as cancer research
[11, 12], microbiology and biotechnology [13-15], pharmaceutical
science [16-19], agricultural science [20-22], materials science
[23-25], dietary supplement [26,27], skin health [28,29], human
health and wellness. The planned to evaluate the impact of the
Biofield Energy Healing Treatment (the Trivedi Effect®) on the test
formulation for antioxidant action concerning lipid peroxidation,
antioxidant activity using standard assays.
Materials and Methods
Chemicals and reagents
Calcitriol, pyridoxine hydrochloride (vitamin B6), beta
carotene (retinol, Provit A), zinc chloride, and magnesium (II)
gluconate were purchased from TCI, Japan. Copper chloride, calcium
chloride, cyanocobalamin (vitamin B12), cholecalciferol (vitamin D3),
sodium carboxymethyl cellulose (Na-CMC), vitamin E (Alpha-Tocopherol),
and iron (II) sulfate were procured from Sigma-Aldrich, USA. Sodium
selenate and ascorbic acid were obtained from Alfa Aesar, India. Panax ginsengextract
and cannabidiol isolate were obtained from Panacea Phytoextracts, India
and Standard Hemp Company, USA, respectively. Other chemicals used in
this experiment were analytical grade procured from India.
Experimental animals
Randomly breed male Sprague Dawley (SD) rats with body
weight ranges from 200 to 300 gm were used in this study. The
animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India.
Animals were randomly divided into nine groups based on their
body weights consist of 6 animals of each group. They were kept
individually in sterilized polypropylene cages with stainless steel
top grill having provision for holding pellet feed and drinking water
bottle fitted with stainless steel sipper tube. The animals were
maintained as per standard protocol throughout the experiment.
Consciousness energy healing strategies
The test formulation was divided into two parts. One part of each
ingredient was considered as the untreated test formulation, where
no Biofield Energy Treatment was provided. Another part of each
ingredient was received Biofield Energy Treatment by Mr. Mahendra
Kumar Trivedi (the Trivedi Effect®) under laboratory conditions for
~3 minutes in the research laboratory, Dabur Research Foundation,
New Delhi, India. Besides, three group of animals were also received
Biofield Energy Treatment under laboratory conditions for ~3
minutes. The energy transmission was done without touching the
samples or animals. Similarly, the control samples were subjected
to “sham” healer under the same laboratory conditions for ~3
minutes for comparison purposes. The “sham” healer did not have
any knowledge about the Biofield Energy Treatment. After that,
the Biofield Energy Treated and untreated test formulations were
kept in the similar sealed condition and used as per the study plan.
The Biofield Energy Treated animals were also be taken back to
experimental room for further proceedings.
Experimental procedure
Seven days after acclimatization, animals were randomized and
grouped based on body weight. All the animals except G1 were fed
with Vitamin D3 deficient diet (VDD) from day -12 to till the end of
the experiment. To induce CYP24A1 expression, to accelerate the
catabolism of endogenous vitamin D3, the rats (Group G2 to G6)
were receive intraperitoneal injections of 40 ng of 19-nor-1,25-
dihydroxyvitamin D2 (Paricalcitol) on days -12, -10, -8, -6, -4, -2,
day 1, 3 and 5. Group G1 to G5 animals were dosed with respective
formulations from Day 1 to till the end of the experiment. However,
Group G6 were not be dosed. Animals (50% of the animals from
each group) were kept for overnight fasting on Day 56 (Tentative).
However, remaining 50% animals were dosed with respective
formulations and were kept for fasting on Day 57 (Tentative) next
day animals were bled and serum was separated for the estimation
of C-reactive protein (CRP). After bleeding, cerebrospinal fluid
(CSF) were collected by standard in-house method using stereotaxic
instrument for the estimation of KLOTHO, Beta-Endorphin,
Serotonin, and 1, 25 (OH)2 D3 by ELISA method.
Estimation of klotho protein, beta-Endorphin, serotonin
and 1, 25 (Oh)2 D3 In cerebrospinal fluids (CSF)
The Klotho protein expression was determined using Rat
Klotho ELISA Kit in rat’s CSF in according to the manufacturer’s
instructions [30].
Assessment of Serum C-reactive protein (CRP)
Serum C-reactive protein were estimated using standard
ELISA assay followed by manufacturer instructions. Serum was
collected from all the animals after completion of the experiment
was examined for level of CRP. The detailed test procedure of the
identification of serum C-reactive protein were performed using
manufactured instructions as per individual ELISA kit. The CRP level
was tested using CUSABIO, ELISA Assay Kit as per manufacturer
instructions.
Statistical analysis
The data were expressed as mean ± Standard Error of Mean
(SEM) and subjected to statistical analysis using Sigma Plot (Version
11.0). For multiple comparison One-way analysis of variance
(ANOVA) followed by post-hoc analysis by Dunnett’s test and for
between two groups comparison Student’s t-test was performed.
The p≤0.05 was considered as statistically significant.
Results and Discussion
Estimation of klotho protein in Cerebrospinal fluids (CSF)
Figure 1: The effect of the test formulation on the level of Klotho protein in cerebrospinal fluids (CSF) in male Sprague Dawley
rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference
item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6:
(VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from
day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9:
(VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are expressed as mean ± SEM, n=6
in each group.
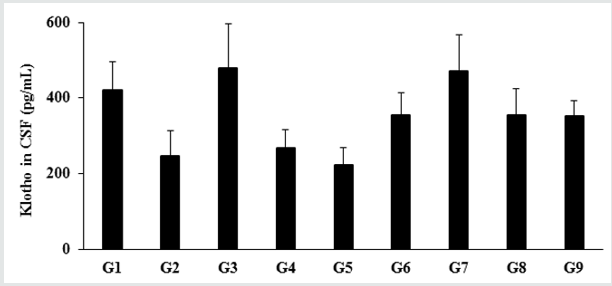
The impact of the test formulation on the expression of Klotho
protein in Cerebrospinal Fluids (CSF) is shown in Figure 1. The level
of Klotho protein in the normal control (G1) group was 419.71 ±
75.81 pg/mL and it was decreased by 41.52% in the disease control
(G2) group (245.43 ± 69.12 pg/mL) induced by vitamin D3 Deficiency
Diet (VDD). The positive control (calcitriol) showed 95.46%
increase the level of Klotho protein expression as compared to the
G2 group. Further, expression of Klotho protein was significantly
increased by 9.31%, 44.24%, 91.97%, 44.24%, and 43.07% in
the untreated test formulation (G4), Biofield Energy Treatment
per se to animals from day -15 (G6), Biofield Energy Treated test
formulation from day -15 (G7), Biofield Energy Treatment per se
plus Biofield Energy Treated test formulation from day -15 (G8),
and Biofield Energy Treatment per se animals plus untreated test
formulation (G9) groups, respectively as compared to the G2 group.
Further, the level of Klotho protein was significantly increased by
31.95%, 75.61%, 31.95%, and 30.88% in the G6, G7, G8, and G9
groups, respectively as compared to the untreated test formulation
group (G4). Klotho protein acts as an anti-aging biomarker. Klotho
gene is recognized as a putative aging-suppressor gene, has a
great interest and provides more useful information of the aging
process. Data obtained from one experiment in mice reported that
the overexpression of the Klotho gene extends the lifespan, and
mutations to the klotho gene which shorten the lifespan [31,32].
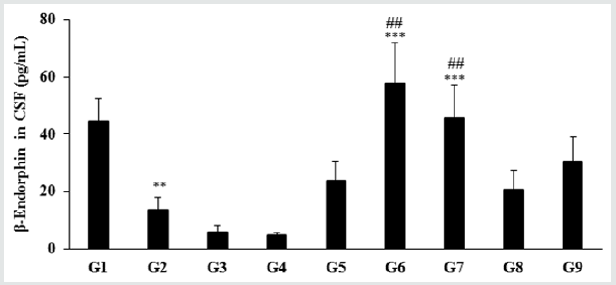
Assessment of CSF biomarker - β-endorphin
The level of β-endorphin in the normal control group (G1) was
44.48 ± 7.87 pg/mL and it was significantly (p≤0.01) decreased by
69.51% in the disease control (G2) group (13.56 ± 4.36 pg/mL)
induced by vitamin D3 Deficiency Diet (VDD). Besides, secretion
of β-endorphin was significantly increased by 75.59%, 325.37%
(p≤0.001), 235.47% (p≤0.001), 52.80%, and 125.88% in the
Biofield Energy Treated test formulation (G4), Biofield Energy
Treatment per se to animals from day -15 (G6), Biofield Energy
Treated test formulation from day -15 (G7), Biofield Energy
Treatment per se plus Biofield Energy Treated test formulation
from day -15 (G8), and Biofield Energy Treatment per se animals plus untreated test formulation (G9) groups, respectively as
compared to the G2 group. Further, the level of β-endorphin was
significantly increased by 418.74%, 1156.64% (p≤0.01), 891.07%
(p≤0.01), 351.42%, and 567.32% in the G5, G6, G7, G8, and G9
groups, respectively as compared to the untreated test formulation
group (G4). β-endorphin is an endogenous opioid neuropeptide and
peptide hormone, considered as cognition, pain and inflammatory
biomarker Figure 2. It is produced in certain neurons within the
central nervous system and peripheral nervous system to relieve
pain when bound to their mu-opioid receptors [33].
Figure 2: The effect of the test formulation on the level of β-endorphin in cerebrospinal fluids (CSF) in male Sprague Dawley rats.
G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference
item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6:
(VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from
day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9:
(VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are expressed as mean ± SEM, n=6
in each group. ***p≤0.001 vs. G2, ##p≤0.01 vs. G4, and **p≤0.01 vs. G1.
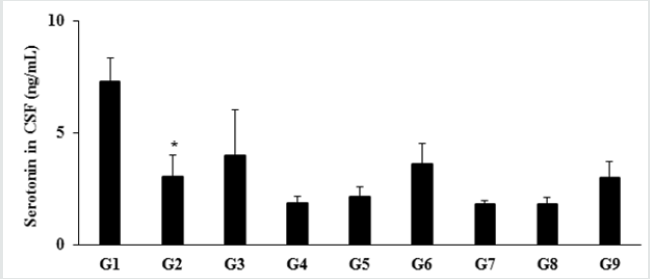
Estimation of 5-hydroxy tryptamine (Serotonin) in CSF
The level of serotonin or 5-hydroxy tryptamine (5-HT) in
the normal control group (G1) was 7.29 ± 1.03 ng/mL and it
was significantly (p≤0.05) decreased by 58.02% in the disease
control (G2) group (3.06 ± 0.93 ng/mL) induced by vitamin D3
Deficiency Diet (VDD). Besides, secretion was increased by 29.41%
and 17.32% in the positive control (G3) and Biofield Energy
Treatment per se to animals from day -15 (G6) groups, respectively
as compared to the G2 group. Further, the level of serotonin was
increased by 16.30%, 95.11% and 63.59% in the G5, G6, and G9
groups, respectively as compared to the untreated test formulation
group (G4). Serotonin (5-HT) in neuron and neurotransmitter loss
leads to aging. The incomplete neurodegenerative processes and
serotonergic neurotransmission also leads to aging process [34]. In
this experiment, the Biofield Energy Treated test formulation had
significantly improve the level of serotonin, which might reduce
aging process Figure 3.
Figure 3: Effect of the test formulation on the level of serotonin in cerebrospinal fluids (CSF) in male Sprague Dawley rats.
G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC); G3: Reference
item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test formulation); G6:
(VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test formulation from
day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9:
(VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are expressed as mean ± SEM, n=6
in each group. *p≤0.05 vs. G1.
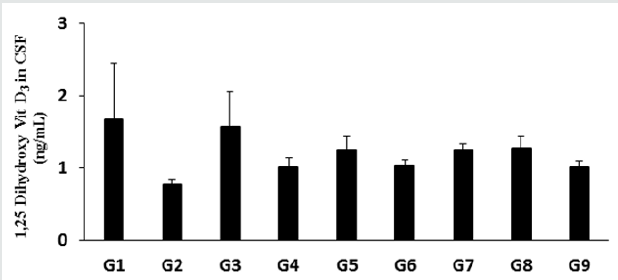
Evaluation of 1, 25 (OH)2 D3 in CSF
The level of 1, 25 (OH)2 D3 in the normal control group (G1) was
1.67 ± 0.78 ng/mL and it was significantly decreased by 53.89%
in the disease control (G2) group (0.77 ± 0.07 ng/mL) induced
by vitamin D3 Deficiency Diet (VDD) is shown in Figure 4. The
positive control group (G3) had significantly increased the level
of 1, 25 (OH)2 D3 by 105.19% compared to the G2 group. Besides,
the level of 1, 25 (OH)2 D3 was significantly increased by 31.17%,
62.34%, 33.77%, 62.34%, 64.94%, and 31.17% in the Biofield
Energy Treated test formulation (G4), Biofield Energy Treated
test formulation (G5), Biofield Energy Treatment per se to animals
from day -15 (G6), Biofield Energy Treated test formulation from
day -15 (G7), Biofield Energy Treatment per se plus Biofield Energy
Treated test formulation from day -15 (G8), and Biofield Energy
Treatment per se animals plus untreated test formulation (G9)
groups, respectively compared to the G2 group. Further, the level of
1, 25 (OH)2 D3was also significantly increased by 23.76%, 23.76%,
and 25.74% in the G5, G7, and G8 groups, respectively.
Figure 4: The effect of the test formulation on the level of 1, 25 (OH)2 D3 in cerebrospinal fluids (CSF) in male Sprague
Dawley rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC);
G3: Reference item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test
formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test
formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from
day -15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are expressed as
mean ± SEM, n=6 in each group.
Effect of the test formulation on serum CRP level
Figure 5: The effect of the Test formulation on change in serum CRP level in vitamin D3 deficiency diet-induced Sprague
Dawley rats. G: Group; G1: Normal control (0.5% CMC); G2: Disease control (VDD: Vitamin D3 deficient diet + 0.5% CMC);
G3: Reference item (VDD + Calcitriol); G4: (VDD + untreated test formulation); G5: (VDD + Biofield Energy Treated test
formulation); G6: (VDD + Biofield Energy Treatment per se to animals from day -15; G7: (VDD + Biofield Energy Treated test
formulation from day -15); G8: (VDD + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day
-15), and G9: (VDD + Biofield Energy Treatment per se animals plus untreated test formulation). Values are expressed as mean
± SEM, n=6 in each group. ***p≤0.001 vs. G1 and G2.
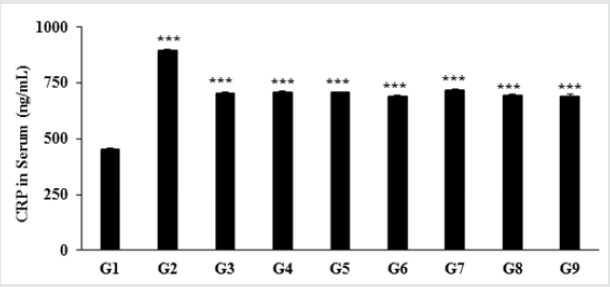
The effect of the novel test formulation on the level of serum
c-reactive protein (CRP) is presented in Figure 1. The serum CRP
level in the disease control (vitamin D3 deficiency) group
was 895.29
± 6.02 ng/mL, which was found to be 98.06% higher than that of the
normal control (G1) group 452.04 ± 3.80 ng/mL. However, calcitriol
group (G3) showed reduced serum CRP level (704.59 ± 6.81 ng/
mL) by 21.30% as compared with the G2 group. The experimental
groups such as untreated test formulation to the untreated animals
(G4) showed reduced CRP level (708.06 ± 6.37 ng/mL) by 20.91%
as compared with the G2 group. Similarly, Biofield Energy Treated
test formulation to the untreated animals (G5) reduced the serum
CRP level (705.79 ± 4.38 ng/mL) by 21.17% as compared to the
G2 group. Biofield Energy Treatment per se to the animals (G6)
reduced the CRP level (688.59 ± 6.46 ng/mL) by 23.09% lower as
compared to the G2 group. In addition, 15 days pre-treatment of
Biofield Energy Treated test formulation (G7) reduced the CRP level
(718.14 ± 2.95 ng/mL) by 19.79% as compared to the G2. Another
group, 15 days pre-treatment of Biofield Energy Treated test
formulation to the Biofield Energy Treated animals (G8) reduced
the CRP level (694.41 ± 5.89 ng/mL) by 22.44% as compared to the
G2. Similarly, the untreated test formulation to the Biofield Energy
Treated animals (G9) reduced the CRP level (688.61 ± 13.29 ng/
mL) by 23.09% as compared to the G2 group. CRP is one of the
major inflammatory biomarkers (highly sensitive protein) for
inflammatory disorders [35,36]. Thus, Biofield Energy Treatment
per se and the test formulation significantly reduced the serum CRP,
which significantly improve the inflammatory conditions Figure 5.
In this research plan, four groups were considered as
preventive maintenance groups. These groups were G6 (Biofield
Energy Treatment per se to animals at -15 days), G7 (Biofield
Energy Treated test formulation from day -15), G8 (Biofield Energy
Treatment per se to animals along with Biofield Treated test
formulation from day -15), and G9 (Biofield treatment per se at
-15 days to animals with untreated test formulation). The results
showed a significant slowdown of disease progression and all other
disease-related symptoms/complications and also reduced the
chances of disease susceptibility in these groups. Specifically, group
G6 (preventive Biofield Energy Treatment group per se at -15 days)
showed the best results as a preventive treatment group compared
to the other groups. Based on the overall data, it suggests that the
Biofield Energy Healing Therapy was found to be most effective and
beneficial to prevent and protect from the occurrence of any type of
disease in the rat model. The data indicated that this therapy could
act as a preventive maintenance therapy to prevent the occurrence
of disease, slow down the disease progression when disease-related
complications are present which will ultimately improve the overall
health and quality of life.
Conclusion
Results of the study revealed that the level of Klotho protein
(anti-aging biomarker) in cerebro-spinal fluids were significantly
increased by 44.2%, 92.0%, 44.2%, and 43.1% in the Biofield
Energy Treatment per se to animals from day -15 (G6), Biofield
Energy Treated test formulation from day -15 (G7), Biofield Energy
Treatment per se plus Biofield Energy Treated test formulation from day -15 (G8), and Biofield Energy Treatment per se animals
plus untreated test formulation (G9) groups, respectively as
compared to the disease control group (G2). Moreover, the level
of β-endorphin (cognition, pain and inflammation biomarker)
was significantly increased by 418.4%, 1155.7% (p≤0.01), 890.4%
(p≤0.01), 351%, and 566.7% in the G5, G6, G7, G8, and G9 groups,
respectively as compared to the G4 group. Moreover, serotonin
level was increased by 94.8%, and 63.4% in the G6 and G9
groups, respectively as compared to the G4. Further, 1, 25 (OH)2
D3 was significantly increased by 61.8%, 33.4%, 61.5%, 64.5%,
and 30.6% in the G5, G6, G7, G8, and G9 groups, respectively as
compared to the VDD induced group (G2). The level of c-reactive
protein (CRP, inflammation biomarker) was reduced by 21.2%,
23.1%, 19.8%, 22.4%, and 23.1% in the G5, G6, G7, G8, and G9
groups, respectively as compared to the G2 group. The current
findings conclude that the Trivedi Effect®-Biofield Energy Healing
Treatment has significantly enhanced the antiaging, cognitive,
and anti-inflammatory biomarkers level that could be helpful in
various aging/psychiatric or inflammatory disorders. which can
also be used to improve the overall health. Biofield Energy Healing
Treatment (The Trivedi Effect®) per se showed the best results with
respect to different beneficial efficacy and biomarker parameters
in the preventive maintenance group, G6, as compared to the other
preventive maintenance groups (G7, G8, and G9) in the rat model
study.
The Biofield Energy Healing Treatment also helped to slow
down the disease progression and disease-related complications
impacting the overall animals’ health. These data suggested that
Biofield Energy Treatment per se and Biofield Energy Treated Test
formulation in combination would be the best treatment strategy
to prevent and protect from the occurrence of any type of disease.
Therefore, the Biofield Energy Healing Treatment (the Trivedi
Effect®) per se might be effective in healthy humans when used as
a preventive maintenance therapy to sustain good health, to boost
overall health, promote healthy aging and increase quality of life. In
the presence of disease, the Biofield Energy therapy might reduce
the severity of any acute/chronic disease (such as auto-immune
related and inflammatory disorders) and / or slow the disease
progression. Thus, the Biofield Energy Treated test formulation
may act as an effective anti-inflammatory and immunomodulatory
product for various autoimmune disorders such as Addison
Disease, Systemic Fibromyalgia, Lupus Erythematosus, Hashimoto
Thyroiditis, Celiac Disease (gluten-sensitive enteropathy), Multiple
Sclerosis, Dermatomyositis, Graves’ Disease, Pernicious Anemia,
Aplastic Anemia, Type 1 Diabetes, Myasthenia Gravis, Crohn’s
Disease, Vasculitis, Scleroderma, Rheumatoid Arthritis, Psoriasis,
Reactive Arthritis, Sjogren Syndrome, Chronic Fatigue Syndrome,
Vitiligo, and Alopecia Areata, as well as inflammatory disorders
such as Irritable Bowel Syndrome (IBS), Asthma, Ulcerative
Colitis, Parkinson’s Disease, Alzheimer’s Disease, Dermatitis,
Atherosclerosis, Hepatitis, and Diverticulitis. Further, the Biofield
Energy Healing Treated test formulation can also be used in the
prevention of immune-mediated tissue damage in cases of organ
transplants like kidney transplants, heart transplants, and liver
transplants, and in the improvement of overall health and quality
of life.